Products
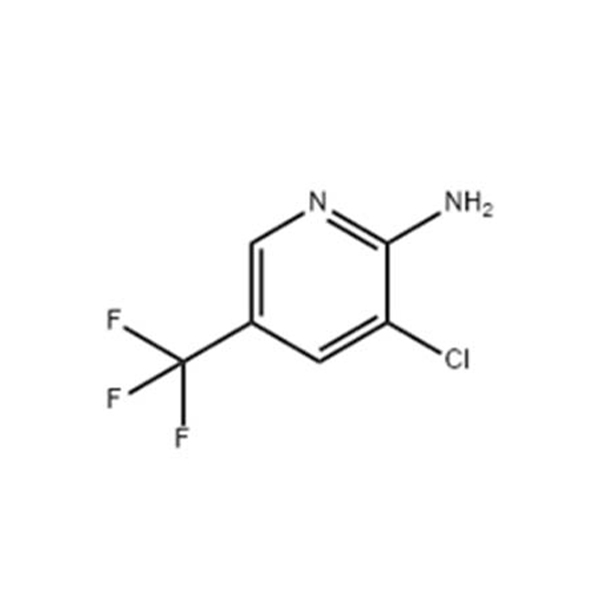
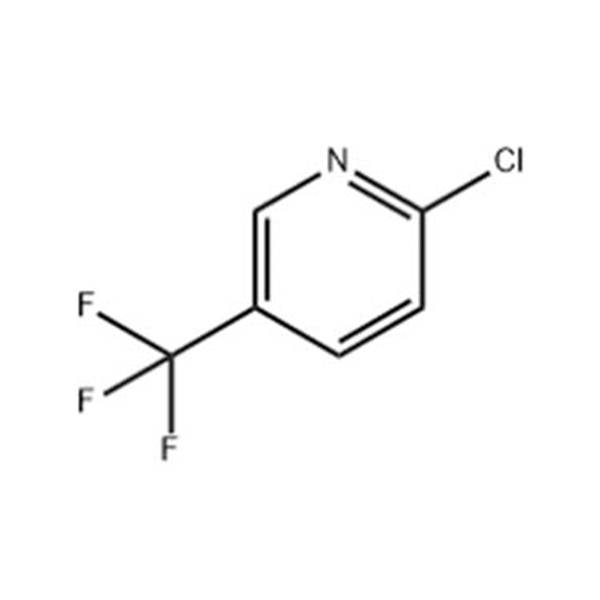
2-Amino-3-chloro-5-trifluoromethylpyridine
CAS: 79456-26-1
Chemical formula : C6H4ClF3N2
Description
marker
2-Amino-3-chloro-5-trifluoromethylpyridine- Introduction
79456-26-1 – Names and Identifiers
|
Name |
2-Amino-3-chloro-5-trifluoromethylpyridine |
|
Synonyms |
2,3,5-actf |
|
CAS |
79456-26-1 |
|
EINECS |
401-670-0 |
|
InChI |
InChI=1/C6H4ClF3N2/c7-4-1-3(6(8,9)10)2-12-5(4)11/h1-2H,(H2,11,12) |
|
InChIKey |
WXNPZQIRDCDLJD-UHFFFAOYSA-N |
79456-26-1 – Physico-chemical Properties
|
Molecular Formula |
C6H4ClF3N2 |
|
Molar Mass |
196.56 |
|
Density |
1.4650 (estimate) |
|
Melting Point |
86-90 °C (lit.) |
|
Boling Point |
205°C |
|
Flash Point |
75.71°C |
|
Water Solubility |
622mg/L at 25℃ |
|
Solubility |
Chloroform (Slightly), Methanol (Slightly) |
|
Vapor Presure |
40.8Pa at 24.85℃ |
|
Appearance |
White crystal |
|
Color |
White to off-white |
|
pKa |
1.79±0.49(Predicted) |
|
Storage Condition |
under inert gas (nitrogen or Argon) at 2–8 °C |
|
Sensitive |
Easily absorbing moisture |
|
Refractive Index |
1.502 |
|
MDL |
MFCD00042154 |
|
Physical and Chemical Properties |
Melting Point: 84-94°C |
79456-26-1 – Risk and Safety
|
Risk Codes |
R22 – Harmful if swallowed |
|
Safety Description |
S61 – Avoid release to the environment. Refer to special instructions / safety data sheets. |
|
WGK Germany |
2 |
|
HS Code |
29339900 |
|
Hazard Class |
IRRITANT |
79456-26-1 – Reference Information
|
LogP |
2.59 at 20℃ |
|
Introduction |
2-amino-3-chloro-5-trifluoromethyl pyridine, as a key intermediate in the preparation of fluroxamine. The production technology of fluxamine is a new technology for the production of a new type of substituted aniline broad-spectrum fungicide in the fine chemical industry of pesticide intermediates, at the same time, the effect of fluoride on a variety of Botrytis cinerea caused by Botrytis cinerea. |
|
preparation |
add 2-aminopyridine (9.4g,1.0mol) to MIBK(48ml), the mixture was stirred until dissolution was clear, and the temperature was lowered to -5 to 0 °c using an ice machine. NBS(18.2g,0.102mol) was dissolved in MIBK (ML) solvent. The NBS solution was added dropwise to the 2-aminopyridine solution at -5~5 ℃ for 1.5-2 hours, the pot temperature was controlled at -5 to 5 ° C. For 4 hours. The 2-aminopyridine content was 0.01% by liquid phase analysis. NCS(15.4g,0.115mol) was dissolved in MIBK(160mL), and the dissolved NCS solution was put into the reactor, and the temperature of the reactor was controlled to 90~100 ℃, The reaction was incubated for 15 hours, and the content of 2-amino-5-bromopyridine was 0.03% by liquid phase analysis. Distillation under reduced pressure, after removing more than 90% of MIBK solvent, add mixed solvent of ethanol (150mL) and water (15mL), heat up to 60~70 ℃, dissolve, recrystallize, filter and rinse, 2-amino-3-chloro-5-bromopyridine is obtained. The above 2-amino-3-chloro-5-bromopyridine solid was added to dioxane (162mL) and heated to 55-60 °c to dissolve, then PdCl2dppf catalyst (0.73g,0.001mol), potassium acetate (14.7g,0.15mol) and ch3bf3 K(19.4g,0.11mol), controlled the kettle temperature at 80~100 ℃ for 12 hours, liquid phase analysis the 2-amino-3-chloro-5-bromopyridine content was 0.05%. The reaction solution is reduced to room temperature, Suction filtration, dioxane washing, distillation of solvent, activated carbon decolorization, Ethanol and heptane (1/5 volume ratio) were slurried and dried to give 2-amino-3-chloro-5-trifluoromethylpyridine (13.9g). |










![[4-(4-Aminophenoxy)(2-pyridyl)]-2-methylcarboxamide](https://www.jihuichem.com/wp-content/uploads/07-1.jpg)
![4-Methyl-3-[[4-(3-pyridinyl)-2-pyrimidinyl]amino]benzoic acid](https://www.jihuichem.com/wp-content/uploads/09-1.jpg)