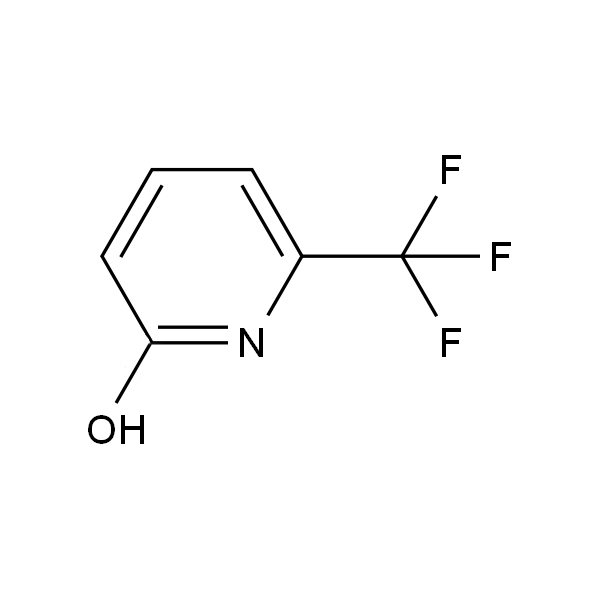
Products
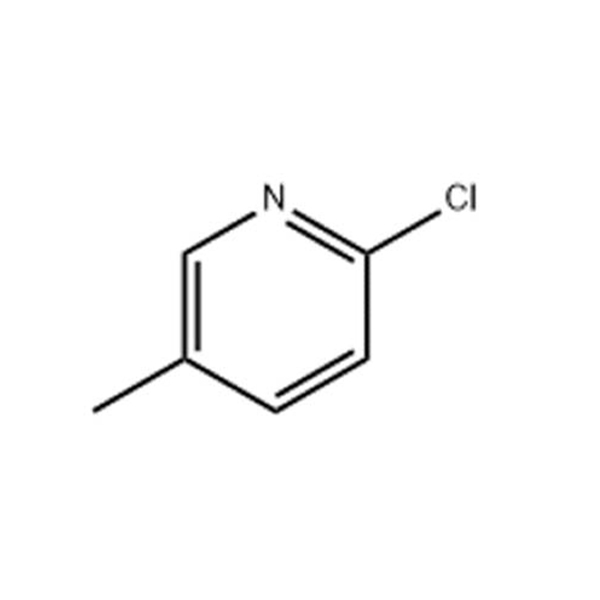
2-Chloro-5-methylpyridine
CAS: 18368-64-4
Molecular Formula: C6H6ClN
Description
marker
2-Chloro-5-methylpyridine – Introduction
18368-64-4 – Names and Identifiers
|
Name |
2-Chloro-5-methylpyridine |
|
Synonyms |
6-CHLORO-3-PICOLINE |
|
CAS |
18368-64-4 |
|
EINECS |
418-050-0 |
|
InChI |
InChI=1/C6H6ClN/c1-5-2-3-6(7)8-4-5/h2-4H,1H3 |
|
InChIKey |
VXLYOURCUVQYLN-UHFFFAOYSA-N |
18368-64-4 – Physico-chemical Properties
|
Molecular Formula |
|
|
Molar Mass |
127.57 |
|
Density |
1.169 g/mL at 25 °C (lit.) |
|
Melting Point |
97 ºC (30 MMHG) |
|
Boling Point |
97 °C/30 mmHg (lit.) |
|
Flash Point |
195°F |
|
Water Solubility |
Slightly soluble in water. |
|
Vapor Presure |
0.736mmHg at 25°C |
|
Appearance |
Bright yellow liquid |
|
Specific Gravity |
1.169 |
|
Color |
Clear light yellow to straw-yellow or light pink-orange |
|
pKa |
0.54±0.10(Predicted) |
|
Storage Condition |
Keep in dark place,Sealed in dry,Room Temperature |
|
Refractive Index |
n20/D 1.53(lit.) |
|
MDL |
MFCD00792460 |
|
Physical and Chemical Properties |
Light yellow liquid |
|
Use |
Used as a pesticide intermediate |
18368-64-4 – Risk and Safety
|
Risk Codes |
R21/22 – Harmful in contact with skin and if swallowed. |
|
Safety Description |
S23 – Do not breathe vapour. |
|
WGK Germany |
2 |
|
RTECS |
US6740000 |
|
HS Code |
29333999 |
18368-64-4 – Reference Information
|
Use |
Used as a pesticide intermediate |
|
production method |
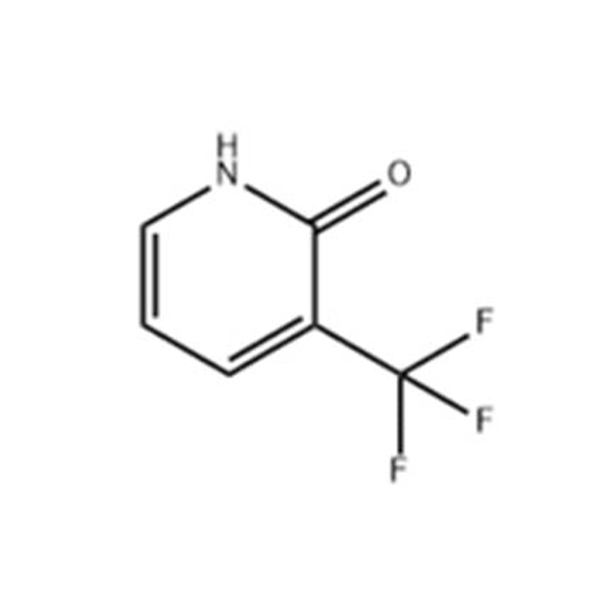
there are many synthesis methods of 2-chloro-5-methylpyridine. according to the different raw materials used, there are mainly the following three routes. Using N-benzyl-N-acrylylacetamide as raw material, N-benzyl-N-acrylylacetamide and phosphorus oxychloride were stirred and reacted at 100 ℃ for 16h to obtain 2-chloro-5-methylpyridine with 67.5% yield. Aminopyridine is used as raw material. The methanol solution of 2-amino-5-methylpyridine is first saturated with hydrogen chloride, and then the mixture of methyl nitrite and hydrogen chloride is introduced to obtain 2-chloro-5-methylpyridine. Methyl nitrite can also be replaced by nitroso chloride or chlorobenzene as a solvent. Using 3-methylpyridine-N-oxide as raw material. Depending on the chlorinating agent used, there are four different ways to choose from. Phosphorus oxychloride is used as chlorinating agent. 3-methylpyridine-N-oxide reacts with phosphorus oxychloride in the presence of an organic base to produce 2-chloro-5-methylpyridine, and has a by-product 2-chloro-3-methylpyridine. Add a certain amount of 3-methylpyridine-N-oxide and solvent into the reaction kettle, add phosphorus oxychloride and organic phosphorus at -10 ℃, after dropping, react at -2 ℃ for 5h, hydrolyze, desolvate, distill to obtain crude products, and then freeze and crystallize to obtain fine monochloride. COCl2 can also be used instead of POCl3 as chlorinating agent. N,N-diethylamino phosphorus oxychloride as chlorinating agent. At room temperature, a dichloromethane solution containing 2-chloro-5-methylpyridine-N-oxide was treated with N,N-diethylaminooxyphosphorus and diisopropylamine dissolved in dichloromethane to obtain a mixture containing 2-chloro-5-methylpyridine-82%, 2-chloro-3-methylpyridine-12% in 83% yields. Phthalyl chloride is used as chlorinating agent. Phthalyl chloride was added dropwise to a mixture containing 3-methylpyridine-N-oxide, triethylamine and dichloromethane for reaction to obtain a mixture containing 2-chloro-5-methylpyridine 84% and 2-chloro-3-methylpyridine 16% with 85% yields. Organic salt is used as intermediate. 2-chloro-5-methylpyridine can also be prepared from n-propionaldehyde, methyl acrylate and morpholine. At present, some domestic enterprises also adopt this method. |










![[4-(4-Aminophenoxy)(2-pyridyl)]-2-methylcarboxamide](https://www.jihuichem.com/wp-content/uploads/07-1.jpg)
![4-Methyl-3-[[4-(3-pyridinyl)-2-pyrimidinyl]amino]benzoic acid](https://www.jihuichem.com/wp-content/uploads/09-1.jpg)