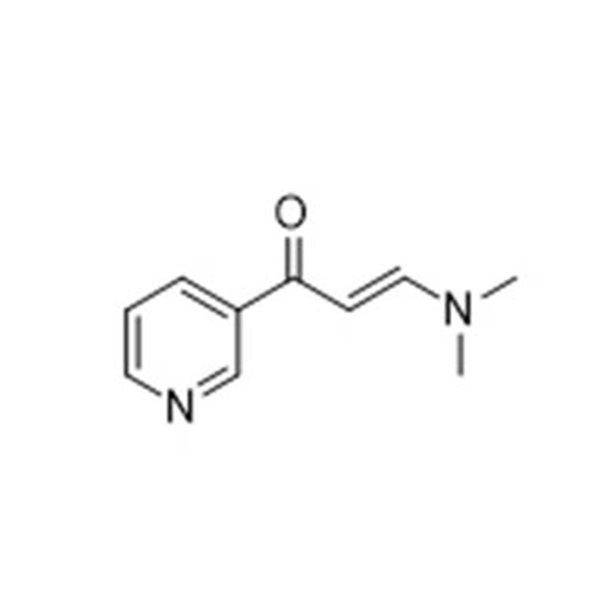
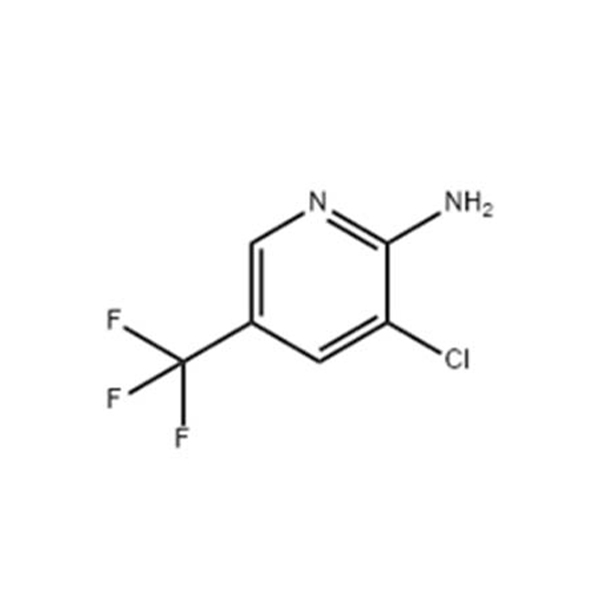
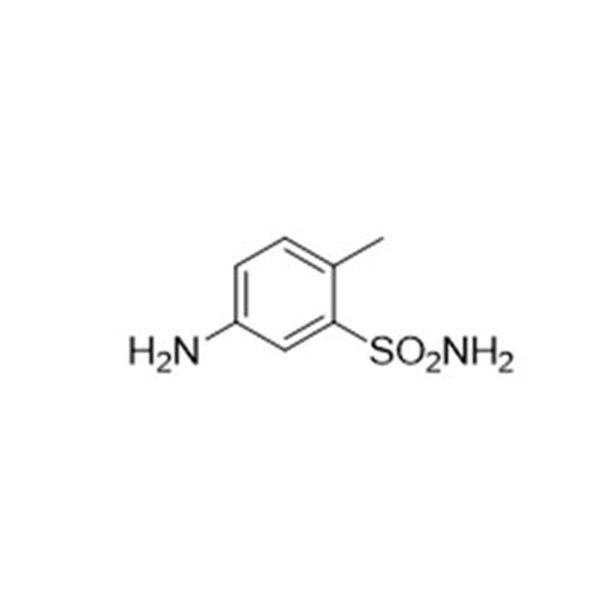
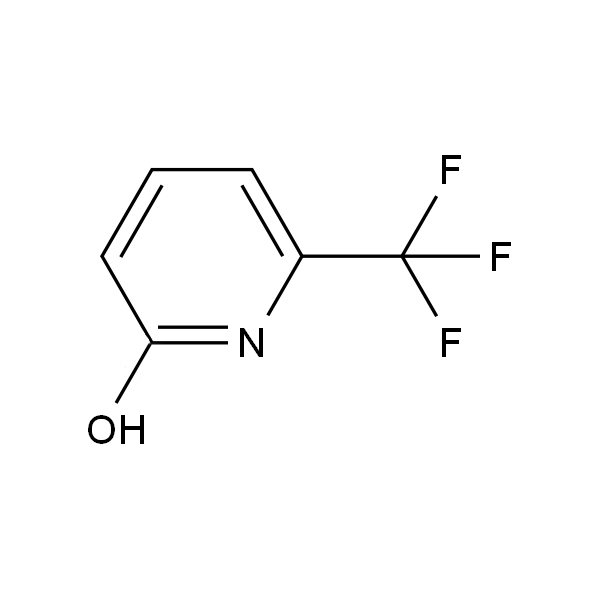
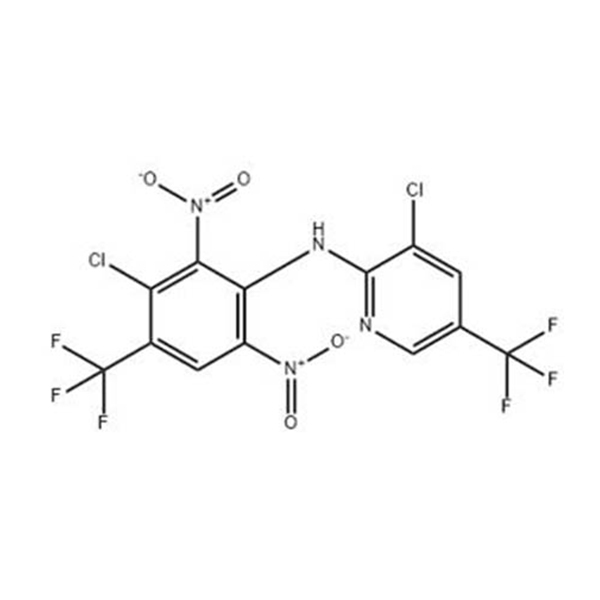
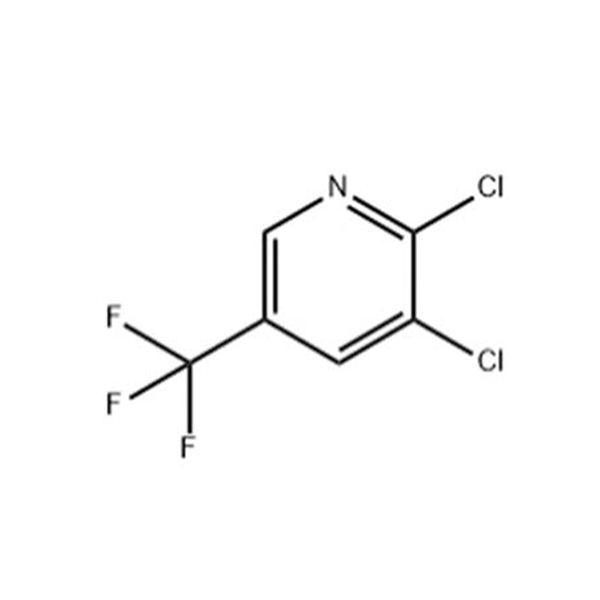
Products
4-Methyl-3-[[4-(3-pyridinyl)-2-pyrimidinyl]amino]benzoic acid
CAS: 641569-94-0
Molecular Formula: C17H14N4O2
Description
marker
4-Methyl-3-[[4-(3-pyridinyl)-2-pyrimidinyl]amino]benzoic acid – Introduction
641569-94-0 – Names and Identifiers
|
Name |
4-Methyl-3-[[4-(3-pyridinyl)-2-pyrimidinyl]amino]benzoic acid |
|
Synonyms |
Nilotinib Intemediate 2 |
|
CAS |
|
|
EINECS |
629-968-8 |
|
InChI |
InChI=1/C17H14N4O2/c1-11-4-5-12(16(22)23)9-15(11)21-17-19-8-6-14(20-17)13-3-2-7-18-10-13/h2-10H,1H3,(H,22,23)(H,19,20,21) |
641569-94-0 – Physico-chemical Properties
|
Molecular Formula |
|
|
Molar Mass |
306.32 |
|
Density |
1.336 |
|
Melting Point |
>257oC (dec.) |
|
Boling Point |
587.9±60.0 °C(Predicted) |
|
Flash Point |
309.367°C |
|
Solubility |
DMSO (Slightly), Methanol (Slightly, Heated) |
|
Vapor Presure |
0mmHg at 25°C |
|
Appearance |
Solid |
|
Color |
Pale Beige |
|
pKa |
4.35±0.10(Predicted) |
|
Storage Condition |
under inert gas (nitrogen or Argon) at 2–8 °C |
|
Stability |
Hygroscopic |
|
Refractive Index |
1.676 |
|
Physical and Chemical Properties |
4-methyl -3-[[4-(3-pyridyl)-2-pyrimidinyl] amino] Benzoic acid is a yellow-white solid with some solubility in methanol and dimethyl sulfoxide. |
641569-94-0 – Reference Information
|
Use |
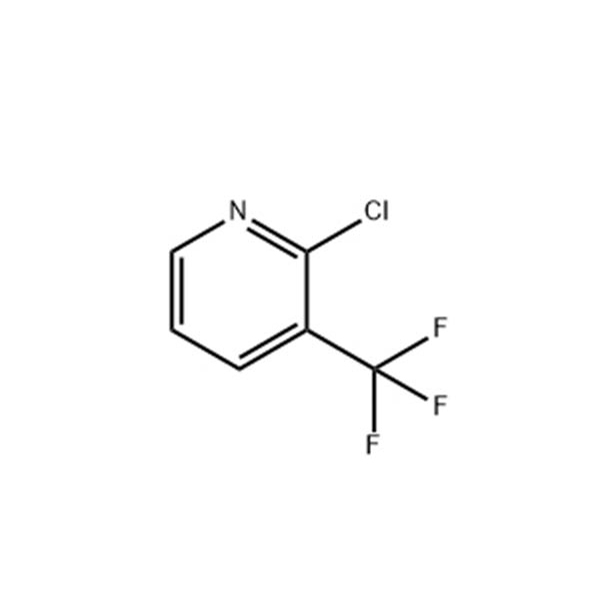
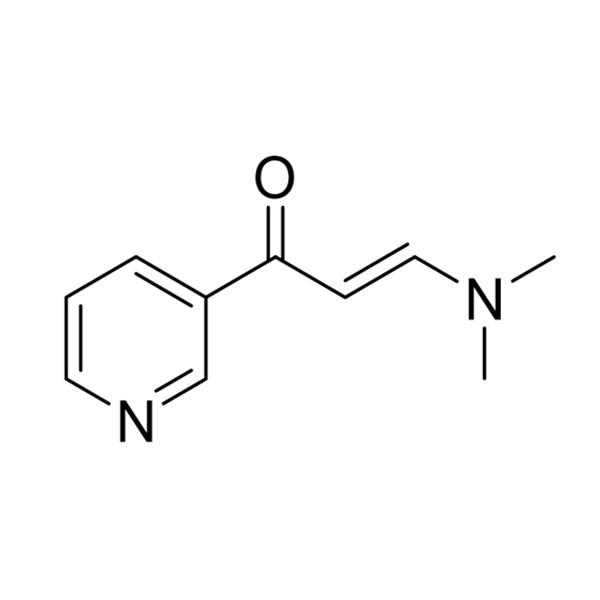
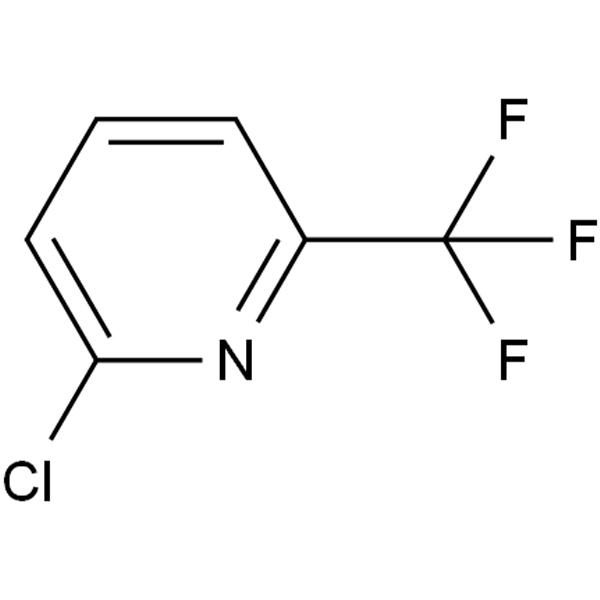
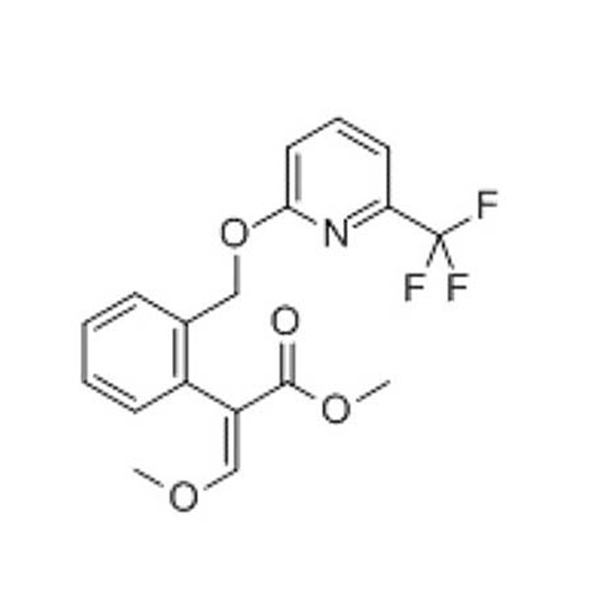
4-methyl -3-[[4-(3-pyridyl)]-2-pyrimidinyl] amino] benzoic acid plays an important role in the synthesis of the antitumor drug molecule nilotinib, however, the carboxyl group in its structure can still be converted into acid chloride and then into ester group by the action of dichlorosulfoxide. |
|
synthetic method |
LiOH (0.24g, 10 mmol, 8 eq.) dissolve in a 3:1 mixture of methanol and water (12 ml) |







![4-Methyl-3-[[4-(3-pyridinyl)-2-pyrimidinyl]amino]benzoic acid](https://www.jihuichem.com/wp-content/uploads/banner4.jpg)
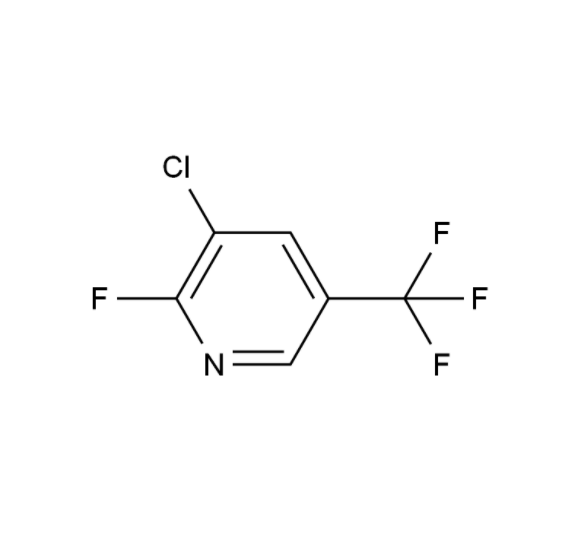


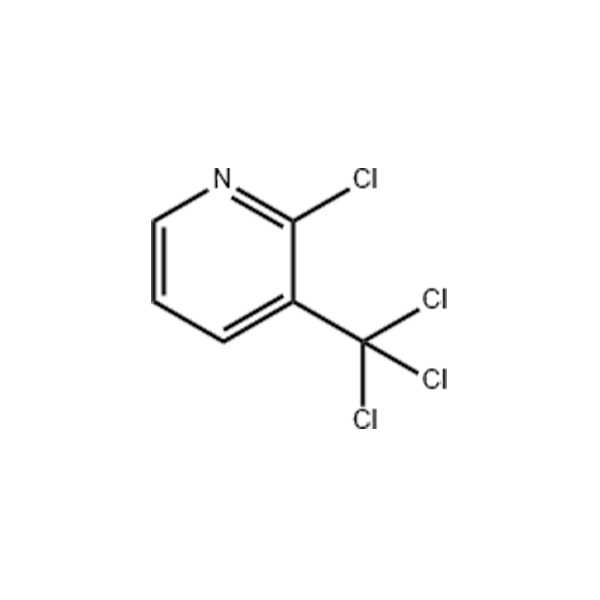
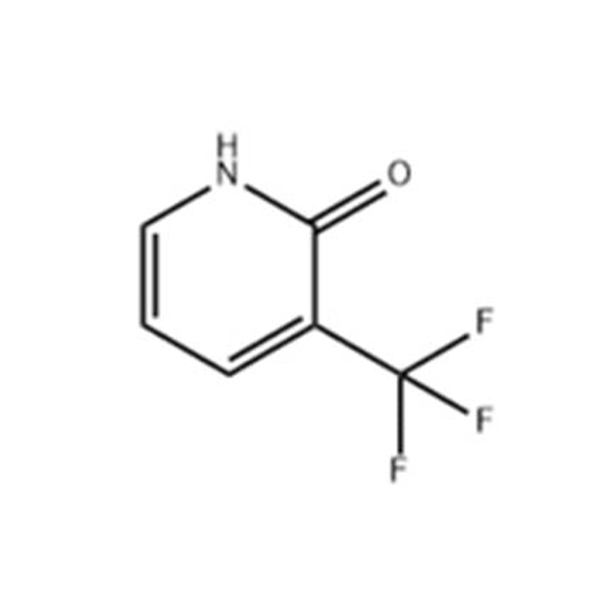
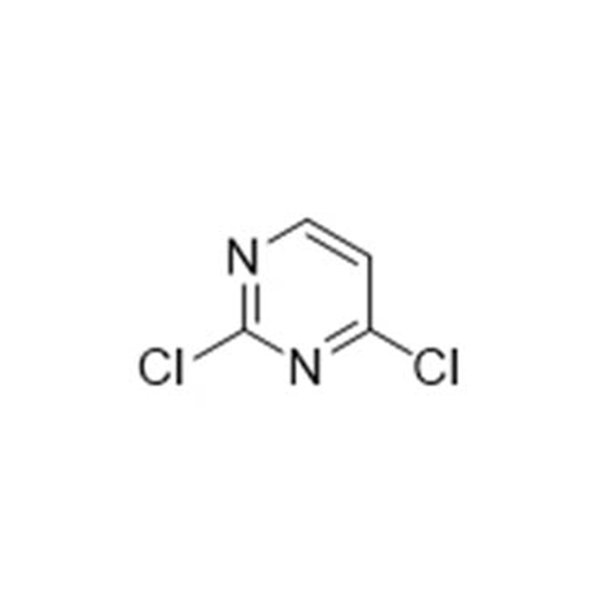
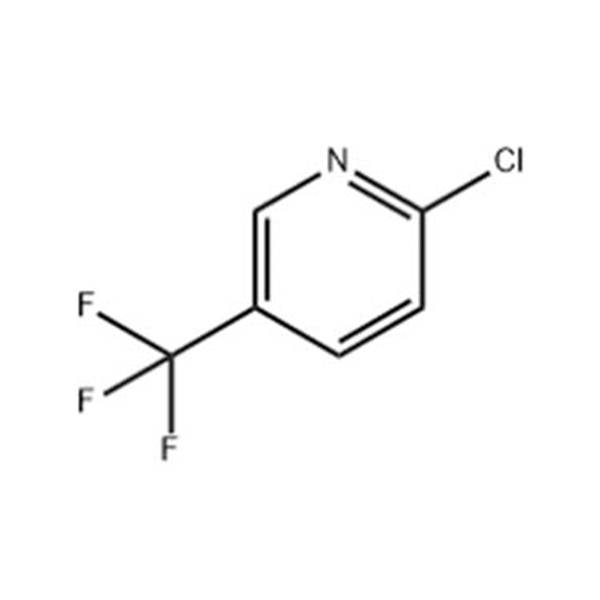
![4-Methyl-3-[[4-(3-pyridinyl)-2-pyrimidinyl]amino]benzoic acid 4-Methyl-3-[[4-(3-pyridinyl)-2-pyrimidinyl]amino]benzoic acid](https://www.jihuichem.com/wp-content/uploads/09-1.jpg)