2-Chloro-3-trifluoromethylpyridine: Properties, Applications & Supplier Guide (2025)
2025-06-13
Introduction
2-Chloro-3-trifluoromethylpyridine is a valuable intermediate widely used in the pharmaceutical, agrochemical, and specialty chemical industries. Known for its halogenated aromatic structure and electron-withdrawing trifluoromethyl group, this compound plays a key role in complex molecule synthesis, especially in the production of biologically active compounds.
In this guide, we cover everything you need to know about 2-Chloro-3-trifluoromethylpyridine, including its chemical properties, synthesis, applications, safety data, and sourcing options.
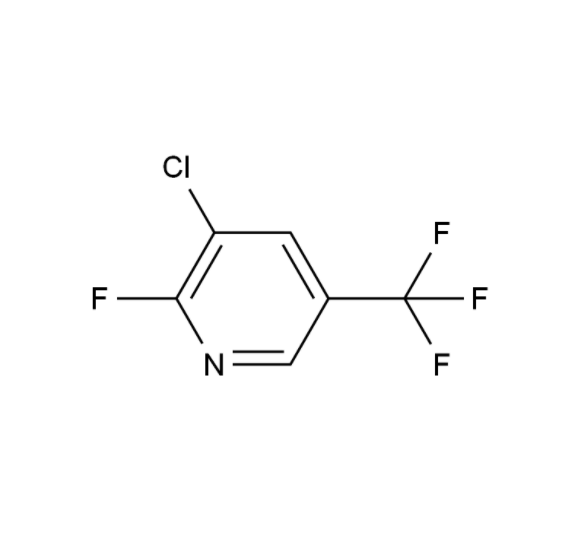
What is 2-Chloro-3-trifluoromethylpyridine?
-
IUPAC Name: 2-Chloro-3-(trifluoromethyl)pyridine
-
CAS Number: 39890-95-4
-
Molecular Formula: C6H3ClF3N
-
Molecular Weight: 181.54 g/mol
-
Chemical Structure:
This compound is a chlorinated pyridine derivative substituted with a trifluoromethyl group at the 3-position. The presence of both Cl and CF₃ groups gives it unique reactivity and stability under various reaction conditions.
Key Physical & Chemical Properties
| Property | Value |
|---|---|
| Appearance | Colorless to pale yellow liquid |
| Boiling Point | ~165–170°C |
| Flash Point | ~63°C |
| Density | ~1.43 g/cm³ |
| Purity | ≥98% (common in commercial supply) |
| Solubility | Soluble in organic solvents (e.g., DCM, MeOH) |
Applications of 2-Chloro-3-trifluoromethylpyridine
This compound is primarily used as a building block in organic synthesis. Its halogen and trifluoromethyl substitutions allow for further derivatization through reactions like:
-
Suzuki, Sonogashira, or Buchwald coupling (via the chloro group)
-
Nucleophilic aromatic substitution (SNAr)
-
Oxidation/reduction of the pyridine ring
-
Trifluoromethylation chemistry
📌 Common Application Areas:
-
Pharmaceuticals
-
Intermediate for developing anti-inflammatory, anti-infective, and anticancer agents.
-
Precursor for heterocyclic compounds with CNS activity.
-
-
Agrochemicals
-
Used in the synthesis of herbicides, insecticides, and fungicides.
-
Enhances biological activity and metabolic stability due to CF₃ group.
-
-
Fluorinated Specialty Chemicals
-
Functionalized fluoropyridines are often required in high-performance materials and advanced intermediates.
-
Synthesis and Reaction Pathways
Typical Synthesis Route:
One common synthetic method involves electrophilic chlorination and selective trifluoromethylation of pyridine derivatives. Industrial production may start from 3-trifluoromethylpyridine, followed by chlorination at the 2-position using reagents like N-chlorosuccinimide (NCS) or SO₂Cl₂ in the presence of a catalyst.
Due to its reactive chloro group, it an excellent intermediate for cross-coupling reactions and other nucleophilic substitutions.
Safety & Handling
| Hazard Class | Irritant, Harmful if Swallowed/Inhaled |
|---|---|
| GHS Labeling | ⚠️ (Exclamation mark symbol) |
| Handling Advice | Use under fume hood, wear gloves/goggles |
| Storage | Store in cool, dry, ventilated area; tightly sealed |
| Incompatible Materials | Strong oxidizers, acids, and bases |
Always consult the latest Safety Data Sheet (SDS) for full regulatory and hazard information.
Global Suppliers & Sourcing Options
When sourcing 2-Chloro-3-trifluoromethylpyridine, it’s essential to verify supplier credibility, purity specifications, and compliance documentation (e.g., COA, SDS, REACH).
🔍 Top Global Suppliers:
-
Merck / Sigma-Aldrich
-
TCI Chemicals
-
Thermo Fisher Scientific
-
Alfa Aesar
-
Biosynth Carbosynth
-
CDMO & CRO partners in China and India
💡 For bulk purchases or custom synthesis, contact manufacturers for sample testing and pricing tiers.
Related Compounds & Derivatives
-
2-Bromo-3-trifluoromethylpyridine (for Suzuki coupling)
-
3-Trifluoromethylpyridine (precursor)
-
2-Amino-3-trifluoromethylpyridine (for pharmaceutical synthesis)
-
Halogenated pyridine derivatives with bioactive scaffolds
Frequently Asked Questions (FAQs)
Q1: Is 2-Chloro-3-trifluoromethylpyridine stable under normal storage conditions?
Yes, when stored properly in a sealed container away from light and moisture, it is stable for long-term use.
Q2: Can I use this compound for Suzuki coupling?
Yes. The chloro substituent at position 2 can participate in palladium-catalyzed cross-coupling reactions.
Q3: What solvents are ideal for reactions using this compound?
Common organic solvents like THF, toluene, DMSO, and acetonitrile are suitable depending on the reaction mechanism.
Conclusion
2-Chloro-3-trifluoromethylpyridine is a versatile and in-demand intermediate for synthetic chemists working in pharmaceuticals, agrochemicals, and fluorinated materials. Its dual halogen-fluoro functionality offers broad reactivity and opens the door for diverse downstream modifications.
Whether you’re developing a new drug candidate or optimizing a reaction for scale-up, this compound offers consistent performance and functional reliability.